1.Why are lithium-ion batteries so powerful?
The performance and reliability of lithium-ion batteries depend to a large extent on the electrolyte. The most commonly used electrolytes today are composed of cyclic and linear carbonates containing soluble lithium salts, typically lithium hexafluorophosphate, which have low viscosity and good ionic conductivity.
However, the electrolyte is unstable at low working potential, and is prone to reductive decomposition on the negative electrode side, resulting in the formation of a solid electrolyte interface (SEI), which has an important impact on battery life and safety. In order to meet the performance requirements of batteries, such as good ion transport performance, stable SEI, long cycle life, etc., electrolyte additives are usually added.
But, due to the wide variety of performance parameters and additives, the selection of electrolyte formulations is very difficult and consumes, many independent experiments and extensive battery performance tests are required. Therefore, how to achieve rapid and accurate screening of electrolyte additives is a difficult problem to be solved at present.
2. Efficient screening methods for additives that you don’t know yet
The researchers found that the interaction between the electrolyte and ionizing radiation can quickly identify the degradation products formed during the battery cycle. The results show that the gas generated by the electrolyte under the action of ionizing radiation is consistent with that generated during the cycle. However, the ionizing radiation test can be completed in only a few hours, which can greatly speed up the screening of electrolyte additives.
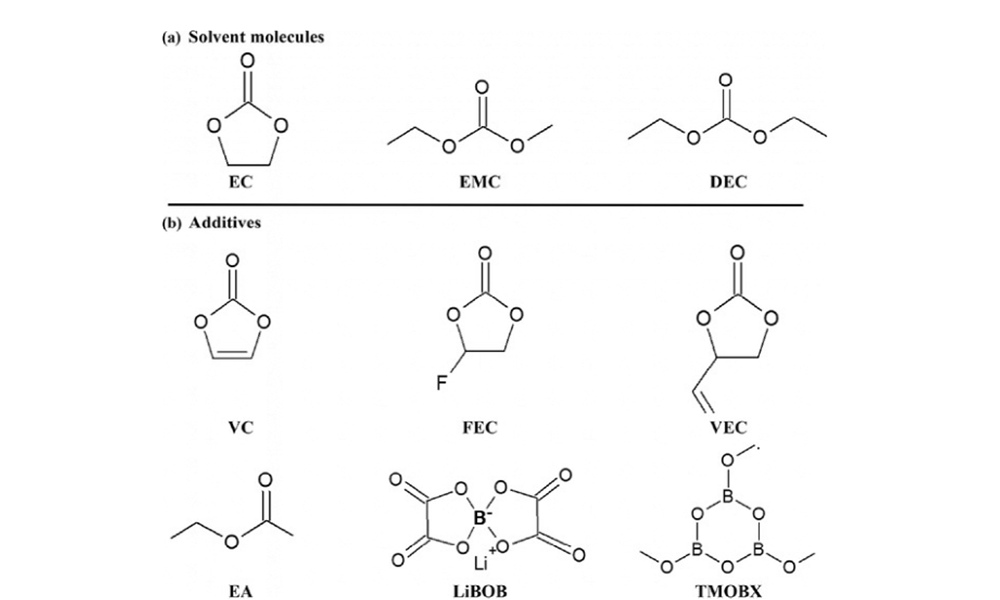
Radiolysis experiments were performed on different electrolytes and additives using a commercial gamma irradiator, and after each irradiation, gas measurements were performed by micro-gas chromatography (µ-GC) to identify and quantify the various gases produced. The control electrolyte is 1M LiPF6 EC/EMC, and the electrolyte solvent and additives are shown in Figure 1.
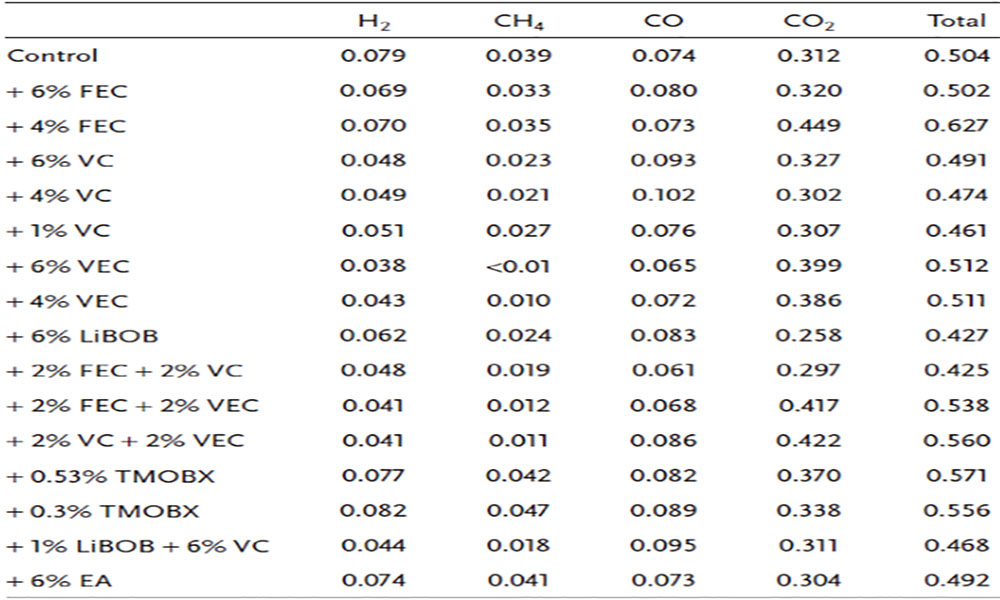
2.1 Generation of H2, CO, CO2 and CH4 gases after irradiation
Under the carbonate-based electrolyte system, H2, CO, CO2, and CH4 dominated the gas produced (about 60%–65% of the total gas production), and the gas production under irradiation was determined by the radiolysis yield ( i.e. the amount of gas produced per unit of energy) is shown in Table 1. The test results show that the total amount of the four gases is not sensitive to the type and concentration of additives, and the difference is not obvious. In addition, among the four gases studied, the amount of CO2 was the largest, the amount of H2 and CO were similar, and the amount of CH4 was the lowest.
In order to judge whether a specific gas can be used as a good indicator to measure the aging degree of the battery during cycling, the authors extracted the H2 radiolysis yield as a function of the nature and amount of the additive, as shown in Figure 2, most of the In all cases, the addition of additives decreased the H2 radiolysis yield, and the trend became more obvious with the increase of additive content. From this result, it can be seen that VC and VEC are the most effective additives for reducing the yield of H2 radiolysis. The carbon-carbon double bond in the additives reacts with hydrogen precursors such as hydrogen atoms, and forms free radical species, thereby reducing the amount of H2 generate.
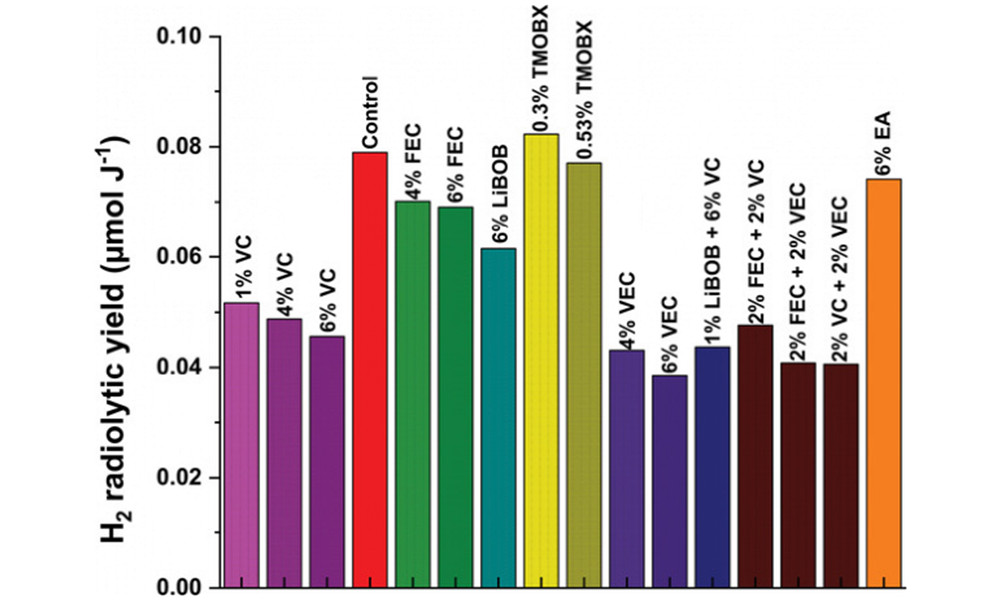
More importantly, the ordering of hydrogen production by irradiation is consistent with the ordering of effects of additives, which demonstrates that irradiation can be used as a predictive tool to screen cell stability through a simple study of electrolyte behavior, especially for H production. sex.
2.2 The effect of linear carbonate on the yield of H2 radiolysis
To investigate the effect of carbonate properties on the yield of H2 radiolysis, the hydrogen production of ethyl methyl carbonate (EMC) and diethyl carbonate (DEC) was investigated in the presence of some additives (VC, FEC, VEC or EA). Happening. It can be seen from Figure 3 that the evolution trends of the H2 radiolysis yields of the two linear carbonates are very similar. Of course, when DEC is used instead of EMC, there is a slight increase in yield due to the presence of the extra -CH2- group. This result also demonstrates that the evolution of the H2 radiolysis yield is due to the nature of the additive rather than the nature of the linear carbonate. Therefore, regardless of the linear carbonate used, the H2 radiolysis yield is a good indicator of electrolyte degradation.
2.3 Influence of water content on the yield of H2 radiolysis
The trace amount of water in the electrolyte can have a large impact on the performance of the battery, therefore, in order to study the effect of water on the H2 radiolysis yield, water was deliberately added to the electrolyte to increase it from 20-30 ppm to 1200 ppm. Except for FEC, the H evolution trends were similar for each additive system at low or high water (Fig. 4).
The yield of H2 radiolysis in the FEC system was increased, even exceeding that of the reference sample, due to the presence of fluorine atoms on the FEC molecules, which resulted in the production of large amounts of HF, which subsequently reacted with the stored glass vials and produced water molecules. Under this vicious circle, the yield of H2 radiolysis was significantly increased. In conclusion, regardless of the water content in the electrolyte (from 20 to 1200 ppm), it did not interfere with the results of the radiolysis tests, demonstrating the high reliability of the method.
2.4The influence of the presence of salt on the gas radiolysis yield
To gain insight into the reaction mechanism, experiments were performed with and without LiPF6 salts and the radiolysis yields were compared in both cases (Fig. 5). The total production of the four gases increased when salt was added, except in the case of FEC (Fig. 5a). This overall enhancement is clearly related to the increase in CO2 (Fig. 5b) in the samples containing LiPF6. In contrast, in the presence of salt, the radiolysis yields of CO and H2 decreased, while the evolution of the methane radiolysis yields depended on the additives, with no clear trend emerging. Furthermore, the presence or absence of lithium salts did not affect the relative magnitude of the H2 radiolysis yields for several additives.
3.Conclusion of the decomposition test method
Ionizing radiation testing proved to be an effective tool for rapid screening of various electrolytes in LIBs. By analyzing the gaseous radiolysis products of these electrolytes, highly reliable results are obtained within a few hours, greatly reducing detection time and eliminating the need for operational testing of the battery. Specifically, the stability of electrolytes can be ranked by the radiolysis yield of H2, and the obtained results are consistent with the test results of JR Dahn and colleagues.
Among all the systems studied, electrolytes containing vinylene carbonate or vinylethylene carbonate exhibited the lowest hydrogen radiolysis yields, a phenomenon attributed to the presence of carbon-carbon double bonds that scavenge hydrogen precursor substance.
Therefore, additives play an important role in LIBs not only to improve the initial quality of the SEI, but also to reduce the formation of gases that can damage the SEI. Additionally, the method is robust across various experimental conditions, such as the presence of traces of water in the electrolyte and the use of different linear carbonates. The researchers also propose a reaction mechanism that explains the formation process of the main molecules in the electrolyte under irradiation.
Radiation chemistry is also applicable to systems other than lithium-ion batteries, facilitating the screening of the best and most efficient electrolytes in novel energy storage batteries such as sodium-ion batteries, supercapacitors, and solid-state batteries.

